MSD Animal Health receives VMD approval for BOVILIS CRYPTIUM®
First vaccine proven to protect calves against Cryptosporidium parvum from the start of colostrum feeding
MILTON KEYNES, UK, 14th August – MSD Animal Health (a division of Merck & Co., Inc., Rahway, N.J., USA (NYSE: MRK)) has announced it has received Veterinary Medicines Directorate (VMD) approval for the first vaccine in Great Britain to protect cattle against the highly infectious parasite that causes cryptosporidiosis, one of the most significant gastrointestinal diseases in cattle1.
BOVILIS CRYPTIUM® is indicated for the active immunisation of pregnant heifers and cows to raise antibodies in colostrum against Gp40 of Cryptosporidium parvum.
“MSD Animal Health is proud to offer this innovative vaccine – a new, science-driven way to combat the devastating parasite C. parvum, which impacts Europe and the rest of the world,” said Philippe Houffschmitt, DVM, MBA, associate vice president of the global ruminant business at MSD Animal Health.
“This novel vaccine offers preventive neonatal protection, which can help preserve cattle well-being from the earliest days of life, as well as help contribute to global food production and safety.”
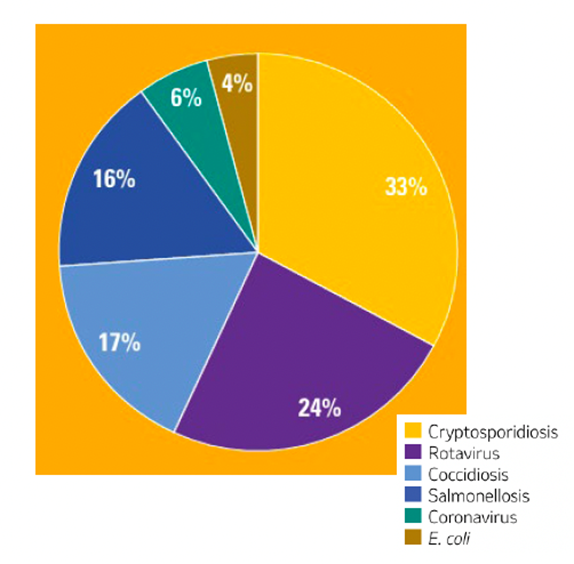
According to Dr Kat Baxter-Smith, veterinary adviser with MSD Animal Health, C. parvum is the most common cause of infectious scour in the UK1 (see figure 1).
“Cryptosporidiosis is widespread on UK dairy and suckler cattle units and is prevalent throughout the year. The disease is mostly seen in calves 7-14 days of age, but can strike at anytime,” she said.
“Infection with the parasite causes blunting of the intestinal villi, reducing capacity for nutrient and water absorption. This has a significant impact on a calf’s future productivity. In a recent UK study2, cryptosporidiosis in the first 16 days of life significantly reduced weight gain over a six month period, with severe disease calves weighing 34 kg less on average than low disease calves. This equated to a £161 reduction in the calf sale price.”
Vaccinating pregnant heifers and cows with BOVILIS CRYPTIUM® can provide protection for calves from birth at the start of colostrum feeding – when they are most vulnerable. Active immunisation raises antibodies in colostrum against C. parvum, which will help to reduce clinical signs (i.e. diarrhoea) when calves are fed this fortified colostrum.
The primary vaccination course is two doses (4 to 5 weeks apart, in the third trimester of pregnancy. To be completed at least 3 weeks before calving). Cattle that have had the primary vaccination course only need a single booster dose during subsequent pregnancies.
“The protection of calves depends on adequate ingestion of colostrum and transition milk from vaccinated cows. It is recommended that all calves are fed colostrum and transition milk during the first five days of life. At least three litres of colostrum should be fed within the first six hours after birth,” said Dr Baxter-Smith.
BOVILIS CRYPTIUM® can be administered to cattle during late pregnancy at the same time as the BOVILIS® ROTAVEC® CORONA vaccine.
“BOVILIS® ROTAVEC® CORONA boosts antibodies in colostrum for other important infectious scour pathogens; rotavirus, coronavirus and both E.coli F5 (K99) and F41. Calves gain protection against these pathogens by drinking the fortified colostrum from their vaccinated mothers,” she added.
Detailed conditions for the use of BOVILISCRYPTIUM® are described in the Summary of Product Characteristics (SPC). Farmers interested in the new BOVILIS CRYPTIUM® vaccine should contact their veterinary professional.
Ends –
References:
- APHA (2019-2023)
- Shaw et al (2020)
BovilisCryptium® contains Cryptosporidium parvum Gp40. POM-V.
Bovilis® Rotavec® Corona contains inactivated rotavirus, coronavirus and E. coli strain CN7985
serotype O101:K99:F41 POM-VPS. Further information is available from the SPC, datasheet or package leaflets. MSD Animal Health UK Limited. Registered office Walton Manor, Walton, Milton Keynes MK7 7AJ, UK. Registered in England & Wales no. 946942. Advice should be sought from the medicine prescriber.
Prescription decisions are for the person issuing the prescription alone.
Use Medicines Responsibly.
© 2024 MSD Animal Health UK Limited. All Rights Reserved.
Figure 1. UK calf scour diagnoses by pathogen (2019 – 2023).
About MSD Animal Health
At MSD, known as Merck & Co., Inc., Rahway, N.J., USA in the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than a century, we’ve been at the forefront of research, bringing forward medicines, vaccines and innovative health solutions for the world’s most challenging diseases. MSD Animal Health, a division of Merck & Co., Inc., Rahway, N.J., USA, is the global animal health business of MSD. Through its commitment to The Science of Healthier Animals®, MSD Animal Health offers veterinarians, farmers, producers, pet owners and governments one of the widest ranges of veterinary pharmaceuticals, vaccines and health management solutions and services as well as an extensive suite of connected technology that includes identification, traceability and monitoring products. MSD Animal Health is dedicated to preserving and improving the health, well-being and performance of animals and the people who care for them. It invests extensively in dynamic and comprehensive R&D resources and a modern, global supply chain. MSD Animal Health is present in more than 50 countries, while its products are available in some 150 markets. For more information, visit www.msd-animal-health.com and connect with us on LinkedIn and X (formerly Twitter).
